FacilityEnsion's biological testing facility is located in Knoxville, Tennessee just minutes away from the University of Tennessee research campus and veterinary school. We maintain a certified biosafety level 2 facility capable of handling human blood borne pathogens. At this facility, we leverage Ension's comprehensive testing capabilities to help medical product developers and manufacturers enhance product performance and improve patient outcomes. Biological TestingAny blood or tissue contacting medical product requires extensive compatibility testing to assess the body's inflammatory response. Ension has developed a range of in vitro and in vivo assays to assess the biocompatibility of a newly designed material or surface treatment. Our team of experienced engineers, surface chemists, and biologists work with the client to design a testing scheme tailored to the medical product's specific performance requirements. We utilize advanced techniques to assess inflammation, coagulation and tissue response at both the material surface and subsequent downstream effects including:
Testing capabilities include:
|
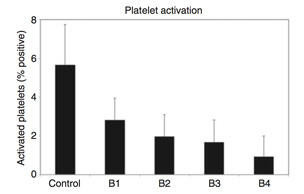
Platelet activation in flowing whole human blood. Surface bound heparin bio-activity levels: Control 1/4 0, B1 1/4 0.45 IU/cm2, B2 1/4 0.81 IU/cm2, B3 1/4 1.18 IU/cm2, and B4 1/4 1.85 IU/cm2; thrombin deactivated.
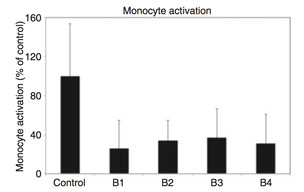
Monocyte activation in flowing whole human blood. Surface bound heparin bio-activity levels: Control 1/4 0, B1 1/4 0.45 IU/cm2, B2 1/4 0.81 IU/cm2, B3 1/4 1.18 IU/cm2, and B4 1/4 1.85 IU/cm2; thrombin deactivated.
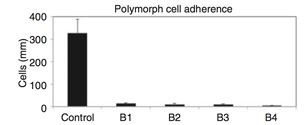
Polymorph adherence to surface in flowing whole human blood. Surface bound heparin bio-activity levels: Control 1/4 0, B1 1/4 0.45 IU/cm2, B2 1/4 0.81 IU/cm2, B3 1/4 1.18 IU/cm2, and B4 1/4 1.85 IU/cm2; thrombin deactivated. |